Under normal circumstances, the anterior chamber is located behind a blood:tissue barrier. The content of plasma proteins within AqH is extremely low (<0.01%), and only T and B lymphocytes are ever detected within the tissues surrounding the anterior chamber. This anatomical barrier represents an important factor in preventing expression of adaptive immunity within the eye. In fact, only when this blood:tissue barrier is breached does the possibility of immune expression in the eye exist. This is because adaptive immune responses are necessarily triggered by antigen-specific T cells and antibodies, both of which are carried in the bloodstream and can only enter the eye from this source.
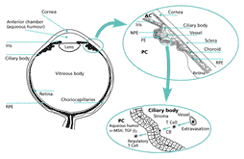
There are also soluble factors within the anterior chamber that act to modify adaptive immune effectors that gain access to this compartment. It has been known for more than a decade that AqH inhibits activation of T lymphocytes in vitro. Since this initial observation, an ever-growing number of soluble factors with this capacity has been identified in AqH (fig. 3). TGF-beta2 and vasoactive intestinal peptide (VIP) are present in AqH at concentrations that completely shut down T-cell proliferation in response to antigen or stimulation with anti-CD3 antibodies. alpha-Melanocyte-stimulating hormone (alpha-MSH) is also present, and although this neuropeptide does not suppress T-cell proliferation, it selectively inhibits activated T cells from producing either IFN-gamma or IL-4. Of even greater interest is the recent discovery by Taylor and his colleagues that immune T cells exposed to antigen in the presence of alpha-MSH are converted into regulator T cells. By secreting TGF-beta, these T cells can suppress the activities of other T cells activated in their microenvironment.
It is important to point out that AqH is not able to prevent cytotoxic T cells from lysing their specific targets. This is important because it indicates that the negative effects of AqH on adaptive immune effectors are not global; rather, they are selective, targeting the T cells most likely to induce inflammation.
AqH also prevents antibodies from triggering complement activation. To date, AqH is known to contain (a) a very small molecular weight factor (<1,000 daltons) that prevents C1q from binding to the Fc portion of IgG antibody molecules, and (b) a larger molecular weight factor (>30,000 daltons) that inhibits the generation of C3b from C3. Once again it is important to note that AqH does not interfere with the effectiveness of antibodies that neutralize viruses, indicating that the immunoglobulin target of AqH factors are adaptive immune effectors (complement-fixing antibodies) that threaten to cause inflammation.
The sources of the factors in AqH that modulate T-cell and antibody functions are not completely defined. Since supernatants of cultures of explanted iris and ciliary body and of cultures of explanted cornea inhibit T-cell activation in a manner similar to AqH it is likely that pigment epithelial cells, as well as corneal endothelium, secrete some of the immunomodulatory factors. Another likely source are terminii of autonomic nerves found within the iris and ciliary body.
Not all local factors that inhibit adaptive immune effectors are soluble. Yoshida and coworkers recently reported that pigment epithelial cells, cultured from the iris and ciliary body, suppress activation of naïve and immune T cells through a direct cell-to-cell contact mechanism. Moreover, T cells that have made contact in this manner with cultured pigment epithelial cells are, on the one hand, spared from apoptosis when given a signal that promotes programmed cell death, and, on the other, converted into regulatory cells that suppress bystander T cells through the secretion of TGF-beta. The cell surface molecules responsible for the interaction between pigment epithelia and T cells remain elusive. Nonetheless, a theme emerges from these considerations.
The ocular microenvironment (anterior chamber) is protected from the ravages of inflammation triggered by adaptive immune effectors at two biologic levels: Architecturally, a blood:tissue barrier acts to limit access to the compartment of blood-borne cells and molecules with receptors specific for antigens expressed locally. Molecularly, the AqH (through soluble factors) and the cells that surround the anterior chamber (through cell surface molecules) prevent adaptive T cells and antibodies from triggering inflammatory cells and molecules directly, and even endow the T cells with the property of further suppressing inflammation. Together, these forces help to explain why it is virtually impossible to elicit delayed hypersensitivity reactions within the anterior chamber of fully sensitized mice, and they make it possible to understand why corneal allografts and xenografts are completely impervious to antibody-mediated rejection mechanisms. These are powerful expressions of the existence of adaptive immune privilege at this site.
The Eye's Dilemma
Immune Privilege
Anterior Chamber of the Eye as an Immune- Privileged Site
Inflammation of Relation to Innate and Adaptive Immune Responses
Ocular Factors That Promote Immune Tolerance of Eye-Derived Antigens
Factors That Modify Expression of Ocular Adaptive Immunity
Innate Immune Privilege in the Eye
Factors That Modify Expression of Innate Ocular Immunity
Clinical Meaning of Ocular Immune Privilege
Selected Reading
Biography
>> next