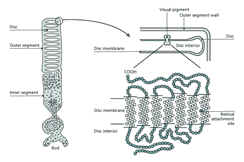
Visual information from the environment is detected by specialized cells called photoreceptors located in a sheet covering the back of the eye. These cells are part of the retina, a thin (ca. 100 µm) layer of cells that is responsible for getting visual information to the brain. Photoreceptors contain two molecules that act together to collect photons. One, opsin, is a protein that sits in a membrane in close association with the other, a visual pigment or chromophore (11-cis-retinal), which is surrounded and held by opsin (fig. 3). When a photon is absorbed by the chromophore, it lengthens by 5 Å by rotating around a double bond. Through this slight transformation, the chromophore makes opsin enzymatically active, ultimately causing, via an amplifying cascade, a decrease in current flow across the outer segment membrane. The main result of this interaction is that the photon energy is transduced into electrical energy which can be interpreted by the nervous system.
The opsins have a family history that precedes eyes as evidenced by comparisons of their DNA. They consist of seven transmembrane helices with short loops on both sides of the membrane. The chromo-phore, retinal, is attached covalently to opsin at a site in the seventh transmembrane domain. These features are common to all metazoan opsins and, based on comparison of the DNA sequences, they must share a common ancestry. In particular, several regions of the molecule show close similarity among opsins from vertebrates, insects and Octopus, whose ancestries diverged in the Cambrian [4]. This homology suggests that the molecule responsible for the initial absorption of photons has been exquisitely tuned over evolutionary time. In addition, the high level of conservation has allowed relatively easy recovery of the cDNAs that encode opsin from the eyes of many different species, giving us a remarkable amount of information on its evolutionary history.
One source of evolutionary information has been the evolution of color vision. There are many selective advantages for animals having color vision including improved detection of food, mates and enemies. To see colors, animals must have photoreceptors sensitive to different wavelengths of light. This is possible through the evolution of slight variants in the opsin molecule through which subtle differences in the amino acids at particular sites 'tune' the chromophore to a particular peak absorbance wavelength. The discovery and clarification of a direct causal link between a molecular structure and its importance for a perceptual process is remarkable in its own right, but also because these features are common to all metazoan opsins. These evolutionary experiments have allowed detailed phylogenetic comparisons, suggesting that vertebrate visual pigments have evolved along at least five lines and diverged from an ancestral type before teleost fish diverged from other vertebrates.
Although metazoan opsins appear to have evolved along several separate lines from a common ancient ancestor, what happened earlier is not clear. Bacteriorhodopsin, from Halobacterium does not show significant amino acid similarity with cattle rhodopsin. Moreover, it is the double bond 13 of the chromophore, rather than 11 that is altered by light. Nonetheless, like metazoan opsins, bacteriorhodopsin belongs to a large superfamily of proteins, all of which have seven transmembrane helices and operate by activating second-messenger cascades. This family of proteins includes neurotransmitter and peptide receptors as well as the family of odorant receptor molecules. Whether similarities within the superfamily result from a very ancient common ancestry or a more recent recruitment is not yet known.
The Evolution of Eyes
Why Do We See What We See?
How Do Eyes Work and How Did They Evolve?
How Do Eyes Capture Photons?
Where Do Lenses Come From?
Eyes: Convergence or Homology?
Conclusions
References
Biography
>> next