|
The Placebo and Nocebo Effect:
How the Therapist’s Words Act on the Patient’s Brain
Fabrizio Benedetti
Department of Neuroscience, University of Turin Medical School,
and National Institute of Neuroscience, Turin, Italy
|
 |
 |
 Clinicians have long known that the context surrounding a therapy is important in any medical treatment and that the words and attitudes of doctors and nurses can have a great impact on the patient. The importance of the verbal interaction between professional personnel and those in their care is illustrated by the emotional impact an anesthetist can have on his or her patient: postoperative pain and narcotic intake were reduced in patients who had been informed about the possible course of their pain following surgery and encouraged to overcome it, compared to a group of patients who had not received a presurgery visit and reassuring words from the anesthetist [1]. Clinicians have long known that the context surrounding a therapy is important in any medical treatment and that the words and attitudes of doctors and nurses can have a great impact on the patient. The importance of the verbal interaction between professional personnel and those in their care is illustrated by the emotional impact an anesthetist can have on his or her patient: postoperative pain and narcotic intake were reduced in patients who had been informed about the possible course of their pain following surgery and encouraged to overcome it, compared to a group of patients who had not received a presurgery visit and reassuring words from the anesthetist [1].
 In another study performed in the 1980s, patients were given different verbal information in general practice consultations. In positive consultations, if no prescription was to be given, they were told that they required none, and if a prescription was to be given, that the therapy would certainly make them better. Conversely, in negative consultations, no firm assurance was given. In fact, if no prescription was to be given, the following statement was made: “I cannot be certain what your problem is, therefore I will give you no treatment.” Conversely, if a prescription was to be given, the patients were told: “I am not sure that the treatment I am going to give you will have an effect.” The treatment was a placebo (an inert substance with no pharmacological action) in both the positive and negative consultations. Two weeks after consultation, recovery was significantly greater in the positive than in the negative group, but there was no difference between the treated and untreated groups, thus indicating that the words the doctor used were critical for recovery [2]. In another study performed in the 1980s, patients were given different verbal information in general practice consultations. In positive consultations, if no prescription was to be given, they were told that they required none, and if a prescription was to be given, that the therapy would certainly make them better. Conversely, in negative consultations, no firm assurance was given. In fact, if no prescription was to be given, the following statement was made: “I cannot be certain what your problem is, therefore I will give you no treatment.” Conversely, if a prescription was to be given, the patients were told: “I am not sure that the treatment I am going to give you will have an effect.” The treatment was a placebo (an inert substance with no pharmacological action) in both the positive and negative consultations. Two weeks after consultation, recovery was significantly greater in the positive than in the negative group, but there was no difference between the treated and untreated groups, thus indicating that the words the doctor used were critical for recovery [2].
 In a similar study, postoperative patients were treated with a painkiller on request, for 3 consecutive days, and with a basal infusion of an inert solution with no pharmacological action (placebo) [3]. However, the symbolic meaning of this basal infusion varied in three different patient groups. The first group was told nothing, the second was told that the infusion could be either a potent analgesic or a placebo, and the third group was told that the infusion was a potent painkiller. Thus the second group received uncertain verbal information (“It can be either a placebo or a painkiller. Thus we are not certain that the pain will subside.”), whereas the third group received clearcut information (“It is a painkiller. Thus pain will subside soon.”). It was found that the intake of the painkiller decreased in the second group compared with the first, and even more in the third group. In fact, the reduction in painkiller requests in the second group was as large as 20.8% compared with the first group, and the reduction in the third group was even larger – 33.8%. It is important to point out that the time course of pain was the same in the three groups over the 3-day period of treatment. Thus the same analgesic effect was obtained with different doses of the painkiller. In a similar study, postoperative patients were treated with a painkiller on request, for 3 consecutive days, and with a basal infusion of an inert solution with no pharmacological action (placebo) [3]. However, the symbolic meaning of this basal infusion varied in three different patient groups. The first group was told nothing, the second was told that the infusion could be either a potent analgesic or a placebo, and the third group was told that the infusion was a potent painkiller. Thus the second group received uncertain verbal information (“It can be either a placebo or a painkiller. Thus we are not certain that the pain will subside.”), whereas the third group received clearcut information (“It is a painkiller. Thus pain will subside soon.”). It was found that the intake of the painkiller decreased in the second group compared with the first, and even more in the third group. In fact, the reduction in painkiller requests in the second group was as large as 20.8% compared with the first group, and the reduction in the third group was even larger – 33.8%. It is important to point out that the time course of pain was the same in the three groups over the 3-day period of treatment. Thus the same analgesic effect was obtained with different doses of the painkiller.
 What all these studies show is that the therapist’s words can be of crucial importance in the therapeutic outcome. They can increase the efficacy of a treatment, can reduce the intake of some drugs, and can improve the patient’s quality of life. Therefore, the therapist’s words, and more generally the psychosocial context around the therapy, may affect both the patient’s mind and body. What all these studies show is that the therapist’s words can be of crucial importance in the therapeutic outcome. They can increase the efficacy of a treatment, can reduce the intake of some drugs, and can improve the patient’s quality of life. Therefore, the therapist’s words, and more generally the psychosocial context around the therapy, may affect both the patient’s mind and body.
Placebo and nocebo effects
 Placebo and nocebo effects represent a very good model to understand how the therapist’s words act on the patient’s brain. A placebo [Lat.: “I shall please”] is a simulation of a medical intervention, be it pharmacological or not, and it has no specific action on the disease to be treated. The placebo effect is the outcome that follows the administration of a placebo. For example, we can simulate an analgesic therapy by giving the patient a sugar pill or a glass of fresh water along with the verbal suggestions that it is a powerful painkiller. The essential point here is that the patient trusts the doctor, believes in the treatment and thus expects a clinical benefit. The placebo effect is, therefore, the effect of the psychosocial context around the therapy, particularly its verbal component, on the patient’s brain (fig. 1). In other words, the psychosocial context may induce expectations of clinical improvement which, in turn, may affect the course of a symptom or a disease. Placebo and nocebo effects represent a very good model to understand how the therapist’s words act on the patient’s brain. A placebo [Lat.: “I shall please”] is a simulation of a medical intervention, be it pharmacological or not, and it has no specific action on the disease to be treated. The placebo effect is the outcome that follows the administration of a placebo. For example, we can simulate an analgesic therapy by giving the patient a sugar pill or a glass of fresh water along with the verbal suggestions that it is a powerful painkiller. The essential point here is that the patient trusts the doctor, believes in the treatment and thus expects a clinical benefit. The placebo effect is, therefore, the effect of the psychosocial context around the therapy, particularly its verbal component, on the patient’s brain (fig. 1). In other words, the psychosocial context may induce expectations of clinical improvement which, in turn, may affect the course of a symptom or a disease.
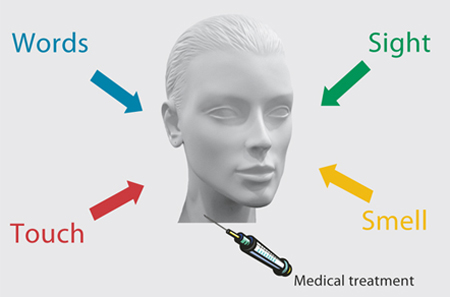
Fig. 1. When a medical treatment, for example the injection of a drug, is administered, there is a complex context around the patient and the therapy which tells the patient that a therapy is being carried out: the sight of the environment, the smell of drugs, the doctor’s words, the touch by needles, and such like. This context may play a crucial role in the therapeutic outcome by inducing expectations of clinical benefit.
|
 The effect of a nocebo [Lat.: “I shall harm”] is the reverse of the placebo effect. Expectations of clinical worsening may induce a real worsening, such as an increase in pain. Therefore, it is important to understand that neither sugar pills nor glasses of water will ever acquire the capacity to heal. What matters are the words that are administered along with the sugar or water. The effect of a nocebo [Lat.: “I shall harm”] is the reverse of the placebo effect. Expectations of clinical worsening may induce a real worsening, such as an increase in pain. Therefore, it is important to understand that neither sugar pills nor glasses of water will ever acquire the capacity to heal. What matters are the words that are administered along with the sugar or water.
 Until about a decade ago, most placebo research employed the methods and techniques of both experimental and social psychology. For example, most research was devoted to an understanding of expectation and conditioning mechanisms. In the first case, it has been shown that complex cognitive factors such as expectation and anticipation of clinical benefit, beliefs, trust and hope, are important and essential in some conditions. In the second case, a mechanism of classical conditioning has been found to play a crucial role in other situations: contextual cues, like the color and shape of pills, may act as a conditioned stimulus that, after repeated associations with an unconditioned stimulus, e.g. the painkiller contained in the pills, is alone capable of inducing analgesia (fig. 2). Until about a decade ago, most placebo research employed the methods and techniques of both experimental and social psychology. For example, most research was devoted to an understanding of expectation and conditioning mechanisms. In the first case, it has been shown that complex cognitive factors such as expectation and anticipation of clinical benefit, beliefs, trust and hope, are important and essential in some conditions. In the second case, a mechanism of classical conditioning has been found to play a crucial role in other situations: contextual cues, like the color and shape of pills, may act as a conditioned stimulus that, after repeated associations with an unconditioned stimulus, e.g. the painkiller contained in the pills, is alone capable of inducing analgesia (fig. 2).
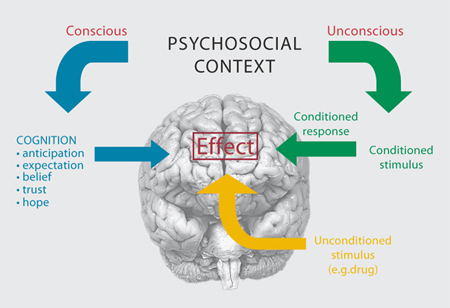
Fig. 2. The psychosocial context may act on the patient’s brain through an unconscious conditioning mechanism whereby, after repeated associations between the context itself (conditioned stimulus) and the drug action (unconditioned stimulus), the context alone may produce an effect. The psychosocial context may also act through complex cognitive factors, such as anticipation and expectation of an outcome, beliefs, trust and hope.
|
 With the advent of modern techniques and methods for investigating the human brain, like neuropharmacology, brain imaging and single-neuron recording in awake patients, neurobiologists became interested in understanding what happens in the brain of subjects who receive a placebo, in other words, who expect a therapeutic benefit. This neurobiological approach is paying dividends, as we are now beginning to understand better the intricate cascade of biological events that take place in the brain during a placebo response. It therefore goes without saying that the placebo/nocebo phenomenon represents an interesting and promising model to clarify some aspects of the mind-body interaction, whereby a complex mental activity, like the expectation of a future outcome, activates specific neuronal systems. With the advent of modern techniques and methods for investigating the human brain, like neuropharmacology, brain imaging and single-neuron recording in awake patients, neurobiologists became interested in understanding what happens in the brain of subjects who receive a placebo, in other words, who expect a therapeutic benefit. This neurobiological approach is paying dividends, as we are now beginning to understand better the intricate cascade of biological events that take place in the brain during a placebo response. It therefore goes without saying that the placebo/nocebo phenomenon represents an interesting and promising model to clarify some aspects of the mind-body interaction, whereby a complex mental activity, like the expectation of a future outcome, activates specific neuronal systems.
Placebos and nocebos move many molecules in the brain
 When we give either a placebo or a nocebo, basically we administer verbal suggestions of either improvement or worsening. Words are very important, and indeed most placebo and nocebo research investigates verbally induced placebo and nocebo responses. However, words are not the only means to induce expectations: the healing environment, the attitudes of the medical personnel, beliefs and trust in medical procedures, can all induce expectations of a therapeutic outcome. When we give either a placebo or a nocebo, basically we administer verbal suggestions of either improvement or worsening. Words are very important, and indeed most placebo and nocebo research investigates verbally induced placebo and nocebo responses. However, words are not the only means to induce expectations: the healing environment, the attitudes of the medical personnel, beliefs and trust in medical procedures, can all induce expectations of a therapeutic outcome.
 The key questions are: What happens in patients’ brains when they expect a clinical improvement or worsening? Are specific brain networks activated? What is the clinical relevance of understanding these mechanisms? The key questions are: What happens in patients’ brains when they expect a clinical improvement or worsening? Are specific brain networks activated? What is the clinical relevance of understanding these mechanisms?
 As far as the mechanisms are concerned, today we know that the administration of placebos and nocebos activates many molecules in the patient’s brain. Most of our knowledge about these mechanisms comes from studies on pain and analgesia, and a summary of the complex cascade of biochemical events following placebo administration and inducing placebo analgesia is provided in figure 3. First of all, there is today general agreement that the endogenous opioid systems play an important role in some circumstances, and several lines of evidence indicate that placebo analgesia is mediated by a pain-modulating network which uses endogenous opioids as neuromodulators. This experimental evidence comes from a combination of both pharmacological and brain-imaging studies. As far as the mechanisms are concerned, today we know that the administration of placebos and nocebos activates many molecules in the patient’s brain. Most of our knowledge about these mechanisms comes from studies on pain and analgesia, and a summary of the complex cascade of biochemical events following placebo administration and inducing placebo analgesia is provided in figure 3. First of all, there is today general agreement that the endogenous opioid systems play an important role in some circumstances, and several lines of evidence indicate that placebo analgesia is mediated by a pain-modulating network which uses endogenous opioids as neuromodulators. This experimental evidence comes from a combination of both pharmacological and brain-imaging studies.
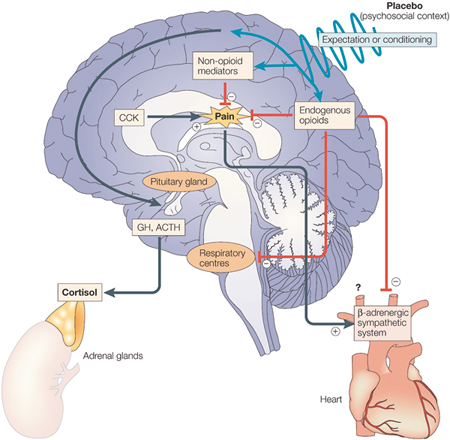
Fig. 3. Cascade of biochemical events that may occur in the brain after placebo administration. Placebo administration, along with verbal suggestions of analgesia (psychosocial context), might reduce pain through opioid and/or nonopioid mechanisms by expectation and/or conditioning mechanisms. The respiratory centers might also be inhibited by endogenous opioids. The β-adrenergic sympathetic system of the heart is also inhibited during placebo analgesia, although the underlying mechanism is not known (either reduction of the pain itself or direct action of endogenous opioids). Cholecystokinin (CCK) counteracts the effects of the endogenous opioids, thereby antagonizing placebo analgesia. Placebos can also act on serotonin-dependent hormone secretion, in both the pituitary and adrenal glands, thereby mimicking the effect of the analgesic drug sumatriptan. ACTH, adrenocorticotrophic hormone; GH, growth hormone. [Reproduced from ref. 4.]
|
 Supporting the involvement of endogenous opioids are a number of pharmacological studies which show that placebo analgesia is antagonized by the opioid antagonist naloxone. In other words, it is possible to prevent the placebo analgesic response by blocking the brain opioid receptors [4]. Using positron emission tomography (PET), a technique that assesses metabolic activity and physiological functions in the brain, it was found that the very same regions in the brain are affected by both a placebo and an opioid drug, thus indicating a related mechanism in placebo-induced and opioid-induced analgesia [5]. In particular, the administration of a placebo induces the activation of the rostral anterior cingulate cortex, the orbitofrontal cortex and the brainstem. Moreover, there is significant covariation in activity between the rostral anterior cingulate cortex and the lower pons/medulla at the level of the rostral ventromedial medulla, and subsignificant covariation between the rostral anterior cingulate cortex and the periacqueductal gray, suggesting that the descending “cingulate cortex/periacqueductal gray/ventromedial medulla” pain-modulating circuit is involved in placebo analgesia (fig. 4). In another study using functional magnetic resonance imaging, a technique similar to PET, brain activation patterns in the prefrontal lobes changed in anticipation of analgesia following placebo administration, and regions involved in pain transmission decreased their activity during the placebo response (fig. 4). Only recently, activation of the endogenous opioid systems by placebo administration was documented directly using PET and in vivo receptor binding in humans [6]. Supporting the involvement of endogenous opioids are a number of pharmacological studies which show that placebo analgesia is antagonized by the opioid antagonist naloxone. In other words, it is possible to prevent the placebo analgesic response by blocking the brain opioid receptors [4]. Using positron emission tomography (PET), a technique that assesses metabolic activity and physiological functions in the brain, it was found that the very same regions in the brain are affected by both a placebo and an opioid drug, thus indicating a related mechanism in placebo-induced and opioid-induced analgesia [5]. In particular, the administration of a placebo induces the activation of the rostral anterior cingulate cortex, the orbitofrontal cortex and the brainstem. Moreover, there is significant covariation in activity between the rostral anterior cingulate cortex and the lower pons/medulla at the level of the rostral ventromedial medulla, and subsignificant covariation between the rostral anterior cingulate cortex and the periacqueductal gray, suggesting that the descending “cingulate cortex/periacqueductal gray/ventromedial medulla” pain-modulating circuit is involved in placebo analgesia (fig. 4). In another study using functional magnetic resonance imaging, a technique similar to PET, brain activation patterns in the prefrontal lobes changed in anticipation of analgesia following placebo administration, and regions involved in pain transmission decreased their activity during the placebo response (fig. 4). Only recently, activation of the endogenous opioid systems by placebo administration was documented directly using PET and in vivo receptor binding in humans [6].
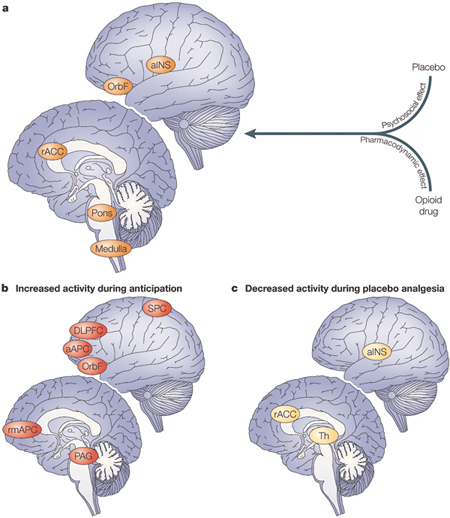
Fig. 4. Summary of brain-imaging studies of placebo analgesia [4]. a. Brain regions activated by the administration of a placebo and the administration of an opioid drug, indicating that mental events (psychosocial effect) and painkillers (pharmacodynamic effect) might have similar effects on the brain. b. During the anticipatory phase, the activated brain regions are likely to represent the activation of a cognitive-evaluative network. c. During placebo analgesia, the activity of different brain areas involved in pain processing decreases, which indicates an effect of the placebo on pain transmission. aAPC, anterior anterior prefrontal cortex; aINS, anterior insula; DLPFC, dorsolateral prefrontal cortex; OrbF, orbitofrontal cortex; PAG, periacqueductal gray; rACC, rostral anterior cingulate cortex; rmAPC, rostral medial anterior prefrontal cortex; SPC, superior parietal cortex; Th, thalamus. [Reproduced from ref. 4.]
|
 The placebo-activated endogenous opioids act not only on pain transmission but on the respiratory centers as well, inducing a placebo respiratory depressant effect, which mimics the typical side effect of opioid drugs. Likewise, placebo-activated endogenous opioids affect the cardiovascular system, slowing down the activity of the heart during placebo analgesia. The placebo-activated endogenous opioids have also been shown to interact with endogenous substances that are involved in pain transmission. In fact, on the basis of the anti-opioid action of cholecystokinin (CCK), CCK-antagonist drugs have been demonstrated to enhance placebo analgesia, suggesting that the placebo-activated opioid systems are counteracted by CCK during a placebo response [4]. The placebo-activated endogenous opioids act not only on pain transmission but on the respiratory centers as well, inducing a placebo respiratory depressant effect, which mimics the typical side effect of opioid drugs. Likewise, placebo-activated endogenous opioids affect the cardiovascular system, slowing down the activity of the heart during placebo analgesia. The placebo-activated endogenous opioids have also been shown to interact with endogenous substances that are involved in pain transmission. In fact, on the basis of the anti-opioid action of cholecystokinin (CCK), CCK-antagonist drugs have been demonstrated to enhance placebo analgesia, suggesting that the placebo-activated opioid systems are counteracted by CCK during a placebo response [4].
 Some types of placebo analgesia appear to be mediated by neuromodulators other than opioids. For example, if a placebo is given after repeated administrations of a nonopioid painkiller, the placebo analgesic response is not mediated by endogenous opioids. In addition, placebo-induced activation of growth hormone and inhibition of cortisol have been described after administration of the analgesic drug sumatriptan, an agonist of serotonin receptors, suggesting that placebos may also act on serotonin-dependent mechanisms [4]. Some types of placebo analgesia appear to be mediated by neuromodulators other than opioids. For example, if a placebo is given after repeated administrations of a nonopioid painkiller, the placebo analgesic response is not mediated by endogenous opioids. In addition, placebo-induced activation of growth hormone and inhibition of cortisol have been described after administration of the analgesic drug sumatriptan, an agonist of serotonin receptors, suggesting that placebos may also act on serotonin-dependent mechanisms [4].
 The role of CCK seems to be particularly important in the nocebo hyperalgesic effect, although nocebo hyperalgesia (i.e. the induction of increased pain) is still little understood. This effect can be blocked by proglumide, a drug that blocks CCK receptors in the brain, indicating that nocebo hyperalgesia is mediated by CCK. Since CCK plays a role in anxiety and a nocebo procedure itself is anxiogenic, these findings imply that proglumide acts on a CCK-dependent increase of anxiety and pain during a nocebo procedure [4]. The role of CCK seems to be particularly important in the nocebo hyperalgesic effect, although nocebo hyperalgesia (i.e. the induction of increased pain) is still little understood. This effect can be blocked by proglumide, a drug that blocks CCK receptors in the brain, indicating that nocebo hyperalgesia is mediated by CCK. Since CCK plays a role in anxiety and a nocebo procedure itself is anxiogenic, these findings imply that proglumide acts on a CCK-dependent increase of anxiety and pain during a nocebo procedure [4].
 Although pain is the best known model to study placebo and nocebo effects, other conditions are now providing further insight into the biological mechanisms of placebos and nocebos. For example, patients who suffer from Parkinson’s disease have been shown to release dopamine after placebo administration [7] and also demonstrated changes in neuronal activity in the basal ganglia (fig. 5) [6]. Similar to the procedure in pain studies, patients were given an inert substance (placebo) and told they were receiving an anti-Parkinsonian drug that would produce an improvement in their motor performance. According to one hypothesis, the placebo-induced release of dopamine in Parkinson’s disease is related to reward mechanisms. In this case, the reward would be the clinical benefit. Although pain is the best known model to study placebo and nocebo effects, other conditions are now providing further insight into the biological mechanisms of placebos and nocebos. For example, patients who suffer from Parkinson’s disease have been shown to release dopamine after placebo administration [7] and also demonstrated changes in neuronal activity in the basal ganglia (fig. 5) [6]. Similar to the procedure in pain studies, patients were given an inert substance (placebo) and told they were receiving an anti-Parkinsonian drug that would produce an improvement in their motor performance. According to one hypothesis, the placebo-induced release of dopamine in Parkinson’s disease is related to reward mechanisms. In this case, the reward would be the clinical benefit.
a
|

|
b
|

|
c
|
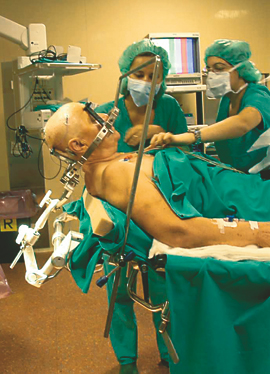
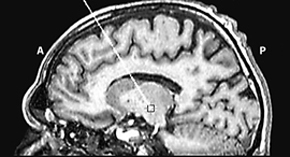
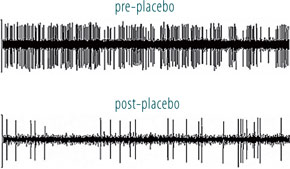
Fig. 5 Recording the activity of single neurons from the brain of an awake patient suffering from Parkinson’s disease. Both the recording apparatus (a) and the electrode track (b) can be seen. In (c), the activity of a single neuron in the subthalamic nucleus can be seen before and after placebo administration.
|
 The neural mechanisms of placebo treatments have also been studied in depression, although the underlying mechanisms are poorly understood [6]. Depressed patients who receive a placebo treatment show both electrical and metabolic changes in the brain. In the first case, placebos induce electroencephalographic changes in the prefrontal cortex of patients with major depression, particularly in the right hemisphere. In the second case, changes in brain glucose metabolism were measured by PET in subjects with unipolar depression. Placebo treatments were associated with metabolic changes in different brain areas. Interestingly, these areas were also affected by the selective serotonin reuptake inhibitor fluoxetine, a result that suggests a possible role for serotonin in placebo-induced antidepressant effects. The neural mechanisms of placebo treatments have also been studied in depression, although the underlying mechanisms are poorly understood [6]. Depressed patients who receive a placebo treatment show both electrical and metabolic changes in the brain. In the first case, placebos induce electroencephalographic changes in the prefrontal cortex of patients with major depression, particularly in the right hemisphere. In the second case, changes in brain glucose metabolism were measured by PET in subjects with unipolar depression. Placebo treatments were associated with metabolic changes in different brain areas. Interestingly, these areas were also affected by the selective serotonin reuptake inhibitor fluoxetine, a result that suggests a possible role for serotonin in placebo-induced antidepressant effects.
Reduced effectiveness of hidden therapies
 Some of the best evidence that expectations affect therapeutic outcome comes from studies on hidden therapies, in which patients do not know that any treatment is being given and thus do not expect any response or result. It is possible to perform a hidden infusion of a drug using a computer-controlled infusion pump which is preprogrammed to deliver the drug at a desired time. The crucial point here is that the patient does not know that any drug is being injected. The computer-controlled infusion pump can deliver a painkiller automatically, without any doctor or nurse in the room, and with the patient completely unaware that a treatment has been started. The outcome following a hidden, or unexpected, treatment is then compared to an open, or expected, treatment. The latter is performed according to routine medical practice, whereby the medical personnel administer a drug along with the reassuring words that the symptom is going to subside shortly [8]. Some of the best evidence that expectations affect therapeutic outcome comes from studies on hidden therapies, in which patients do not know that any treatment is being given and thus do not expect any response or result. It is possible to perform a hidden infusion of a drug using a computer-controlled infusion pump which is preprogrammed to deliver the drug at a desired time. The crucial point here is that the patient does not know that any drug is being injected. The computer-controlled infusion pump can deliver a painkiller automatically, without any doctor or nurse in the room, and with the patient completely unaware that a treatment has been started. The outcome following a hidden, or unexpected, treatment is then compared to an open, or expected, treatment. The latter is performed according to routine medical practice, whereby the medical personnel administer a drug along with the reassuring words that the symptom is going to subside shortly [8].
 In postoperative pain following oral surgery, a hidden injection of 6–8 mg of morphine was found to correspond to an open injection of placebo. In other words, telling a patient that a painkiller is being injected (actually a placebo) is as potent as 6–8 mg of morphine. An analgesic effect stronger than the placebo was only observed when the hidden morphine dose was increased to 12 mg. This suggests that an open injection of morphine in full view of the patient, which is the usual medical practice, is more effective than a hidden injection, because in the latter, the placebo component is absent [4]. An analysis of the differences between open and hidden injections in the postoperative setting has been performed recently. The effects of four widely used painkillers (buprenorphine, tramadol, ketorolac, metamizol) were analyzed following either open or hidden injections. The analgesic dose needed to reduce the pain by 50% was much higher with hidden infusions than with open ones for all four painkillers, indicating that a hidden administration is less effective than an open one. The time course of postoperative pain was also found to be significantly different between open and hidden injections. In fact, during the first hour after the injection, pain ratings were much higher with a hidden injection than with an open one (fig. 6) [8]. In postoperative pain following oral surgery, a hidden injection of 6–8 mg of morphine was found to correspond to an open injection of placebo. In other words, telling a patient that a painkiller is being injected (actually a placebo) is as potent as 6–8 mg of morphine. An analgesic effect stronger than the placebo was only observed when the hidden morphine dose was increased to 12 mg. This suggests that an open injection of morphine in full view of the patient, which is the usual medical practice, is more effective than a hidden injection, because in the latter, the placebo component is absent [4]. An analysis of the differences between open and hidden injections in the postoperative setting has been performed recently. The effects of four widely used painkillers (buprenorphine, tramadol, ketorolac, metamizol) were analyzed following either open or hidden injections. The analgesic dose needed to reduce the pain by 50% was much higher with hidden infusions than with open ones for all four painkillers, indicating that a hidden administration is less effective than an open one. The time course of postoperative pain was also found to be significantly different between open and hidden injections. In fact, during the first hour after the injection, pain ratings were much higher with a hidden injection than with an open one (fig. 6) [8].
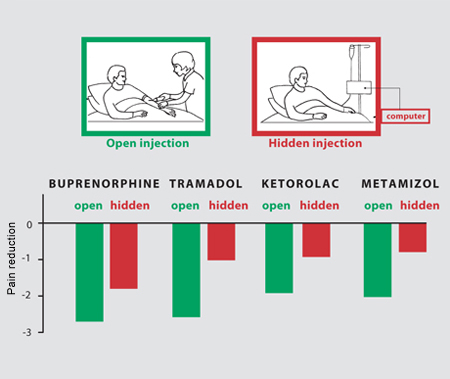
Fig. 6. An open injection is compared to a hidden injection of one of four painkillers. An open injection is performed by a doctor in full view of the patient, whereas a hidden injection is carried out by a computer with the patient completely unaware that a drug is being administered. In all cases, a hidden injection is less effective than an open one.
|
 Similar results have been obtained in other conditions, such as anxiety and Parkinson’s disease, indicating that pain is not a special case. Therefore, knowledge about a therapy by patients can make a difference, as it may affect the therapeutic outcome [8]. Similar results have been obtained in other conditions, such as anxiety and Parkinson’s disease, indicating that pain is not a special case. Therefore, knowledge about a therapy by patients can make a difference, as it may affect the therapeutic outcome [8].
The clinical impact
 There are many clinical implications of these recent advances in the neurobiology of the placebo/nocebo effect. First of all, when we want to assess the efficacy of a new drug in a clinical trial, it is necessary to take certain points into consideration. First, we need to address the expectations of a patient in a clinical trial through the study of perceived assignment to a group (either placebo or active treatment) rather than the standard analysis of actual assignment. In other words, the patient’s perceived assignment to a group in a clinical trial may have a greater impact on the outcome than the actual treatment itself. There are many clinical implications of these recent advances in the neurobiology of the placebo/nocebo effect. First of all, when we want to assess the efficacy of a new drug in a clinical trial, it is necessary to take certain points into consideration. First, we need to address the expectations of a patient in a clinical trial through the study of perceived assignment to a group (either placebo or active treatment) rather than the standard analysis of actual assignment. In other words, the patient’s perceived assignment to a group in a clinical trial may have a greater impact on the outcome than the actual treatment itself.
 Second, any pharmacological agent may interfere with the cascade of biochemical events triggered by expectations. In a clinical trial carried out in the 1990s, a group taking a placebo was compared with a group taking the CCK-antagonist, proglumide. The analgesic effect was greater in the proglumide than in the placebo group, suggesting that proglumide is a good analgesic. However, this conclusion is erroneous, because a hidden injection of proglumide is totally ineffective, demonstrating that it is not an analgesic, but that it enhances placebo-activated endogenous opioids. This finding means that, in light of the fact that some substances may interfere with placebo-activated endogenous opioids, we must consider that a new drug (like a CCK-antagonist) may have no analgesic properties in and of itself but may enhance placebo-activated endogenous opioids [4]. Second, any pharmacological agent may interfere with the cascade of biochemical events triggered by expectations. In a clinical trial carried out in the 1990s, a group taking a placebo was compared with a group taking the CCK-antagonist, proglumide. The analgesic effect was greater in the proglumide than in the placebo group, suggesting that proglumide is a good analgesic. However, this conclusion is erroneous, because a hidden injection of proglumide is totally ineffective, demonstrating that it is not an analgesic, but that it enhances placebo-activated endogenous opioids. This finding means that, in light of the fact that some substances may interfere with placebo-activated endogenous opioids, we must consider that a new drug (like a CCK-antagonist) may have no analgesic properties in and of itself but may enhance placebo-activated endogenous opioids [4].
 I believe that the trial described above exposes an urgent need to understand the neurobiological mechanisms of the placebo response. By borrowing the Heisenberg uncertainty principle from physics, which imposes limits on the precision of a measurement, we can apply a similar principle to the outcomes of clinical trials. In the same way that the uncertainty principle states that a dynamic disturbance is necessarily induced in a system by a measurement, a dynamic disturbance might be induced in the brain in clinical trials by almost any type of drug. The nature of this dynamic disturbance is the interference of the injected drug with the expectation biochemical pathways, with an effect on both the outcome measures and the interpretation of the data. One possible solution to this problem is a hidden injection of the drug to be tested, in order to eliminate all the biochemical events triggered by expectations. I believe that the trial described above exposes an urgent need to understand the neurobiological mechanisms of the placebo response. By borrowing the Heisenberg uncertainty principle from physics, which imposes limits on the precision of a measurement, we can apply a similar principle to the outcomes of clinical trials. In the same way that the uncertainty principle states that a dynamic disturbance is necessarily induced in a system by a measurement, a dynamic disturbance might be induced in the brain in clinical trials by almost any type of drug. The nature of this dynamic disturbance is the interference of the injected drug with the expectation biochemical pathways, with an effect on both the outcome measures and the interpretation of the data. One possible solution to this problem is a hidden injection of the drug to be tested, in order to eliminate all the biochemical events triggered by expectations.
 Besides this impact on clinical trials, there are also important implications for routine medical practice. The studies on hidden therapies teach us that the knowledge about a therapy affects the therapeutic outcome. Therefore, clinicians should strive to communicate their therapeutic interventions to their patients in order to increase expectations and to trigger the activation of those molecules in the brain that mediate placebo responses. Interestingly, a disruption of expectation/placebo-related analgesic mechanisms may occur in a clinical condition, Alzheimer’s disease, in which an impairment of cognition is associated with the loss of connectivity among different brain regions, particularly the frontal lobes. Alzheimer patients with frontal lobe impairment show reduced expectations and placebo effects, so that analgesic therapies have been found to be less effective [9]. These findings underscore the urgent need to consider a possible revision of the therapeutic approach in Alzheimer patients, such as a dose increase to compensate for the loss of the endogenous expectation and placebo mechanisms. Besides this impact on clinical trials, there are also important implications for routine medical practice. The studies on hidden therapies teach us that the knowledge about a therapy affects the therapeutic outcome. Therefore, clinicians should strive to communicate their therapeutic interventions to their patients in order to increase expectations and to trigger the activation of those molecules in the brain that mediate placebo responses. Interestingly, a disruption of expectation/placebo-related analgesic mechanisms may occur in a clinical condition, Alzheimer’s disease, in which an impairment of cognition is associated with the loss of connectivity among different brain regions, particularly the frontal lobes. Alzheimer patients with frontal lobe impairment show reduced expectations and placebo effects, so that analgesic therapies have been found to be less effective [9]. These findings underscore the urgent need to consider a possible revision of the therapeutic approach in Alzheimer patients, such as a dose increase to compensate for the loss of the endogenous expectation and placebo mechanisms.
 Understanding the biochemical bases of the nocebo effect has important implications as well. First, inducing negative expectations may worsen some symptoms and may interfere with recovery from a disease. Second, the identification of a neuromodulator of nocebo hyperalgesia, i.e. CCK, may lead to the development of new CCK-antagonists for the treatment of anxiety-related pain. Likewise, understanding the nocebo effect in other conditions may lead to new therapeutic strategies for various diseases. Understanding the biochemical bases of the nocebo effect has important implications as well. First, inducing negative expectations may worsen some symptoms and may interfere with recovery from a disease. Second, the identification of a neuromodulator of nocebo hyperalgesia, i.e. CCK, may lead to the development of new CCK-antagonists for the treatment of anxiety-related pain. Likewise, understanding the nocebo effect in other conditions may lead to new therapeutic strategies for various diseases.
The future
 The future challenge for placebo research is to expand our knowledge about placebo- and nocebo-related phenomena in different diseases, and in particular to refine our understanding about where, when and how placebos and nocebos act. This knowledge will provide us with important information on the functioning of our brain and body as well as on the possible implications and applications in the clinical setting. In the first case, the placebo/nocebo phenomenon promises to shed new light on the interaction between mind, brain and the body. In the second case, better neurobiological understanding may lead to improvements in clinical practice, including the therapist-patient interaction and different psychotherapeutic approaches. The future challenge for placebo research is to expand our knowledge about placebo- and nocebo-related phenomena in different diseases, and in particular to refine our understanding about where, when and how placebos and nocebos act. This knowledge will provide us with important information on the functioning of our brain and body as well as on the possible implications and applications in the clinical setting. In the first case, the placebo/nocebo phenomenon promises to shed new light on the interaction between mind, brain and the body. In the second case, better neurobiological understanding may lead to improvements in clinical practice, including the therapist-patient interaction and different psychotherapeutic approaches.
 Finally, we need to explore further the impact of placebo research on society in order to identify both the positive and negative aspects of the suggestibility of the human mind. If future research leads to a full understanding of the mechanisms of psychological suggestibility, an ethical debate will then be required to prevent the misuse of placebos and nocebos. There are, therefore, potentially negative outcomes of placebo research that need to be discussed and considered from an ethical perspective. I believe that these issues are worthy of intense scientific scrutiny and will lead to fundamental insights into human biology. Finally, we need to explore further the impact of placebo research on society in order to identify both the positive and negative aspects of the suggestibility of the human mind. If future research leads to a full understanding of the mechanisms of psychological suggestibility, an ethical debate will then be required to prevent the misuse of placebos and nocebos. There are, therefore, potentially negative outcomes of placebo research that need to be discussed and considered from an ethical perspective. I believe that these issues are worthy of intense scientific scrutiny and will lead to fundamental insights into human biology.
References

|
| 1 |
|
Egbert LD, Battit GE, Welch CE, Bartlett MK: Reduction of postoperative pain by encouragement and instruction of patients.
N Engl J Med 1964;270:825–827.

|
| 2 |
|
Thomas KB: General practice consultations: is there any point in being positive?
BMJ 1987;294:1200–1202.

|
| 3 |
|
Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F: Response expectancies in placebo analgesia and their clinical relevance.
Pain 2001;93:77–84.

|
| 4 |
|
Colloca L, Benedetti F: Placebos and painkillers: is mind as real as matter?
Nat Rev Neurosci 2005;6:545–552.

|
| 5 |
|
Petrovic P, Kalso E, Petersson KM, Ingvar M: Placebo and opioid analgesia – imaging a shared neuronal network.
Science 2002;295:1737–1740.

|
| 6 |
|
Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK: Neurobiological mechanisms of the placebo effect.
J Neurosci 2005;25:10390–10402.

|
| 7 |
|
de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ: Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease.
Science 2001;293:1164–1166.

|
| 8 |
|
Colloca L, Lopiano L, Lanotte M, Benedetti F: Overt versus covert treatment for pain, anxiety and Parkinson’s disease.
Lancet Neurol 2004;3:679–684.

|
| 9 |
|
Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, Asteggiano G: Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective.
Pain 2006;121:133–144.

|

Fabrizio Benedetti obtained his MD degree at the University of Turin Medical School in Italy in 1981. He joined the Postdoctoral School of Psychiatry and Biobehavioral Sciences at the University of California in Los Angeles where he won the Silbert International Award. In the 1990s he was appointed Assistant Professor at the Southwestern Medical Center of the University of Texas in Dallas. He is now Professor of Physiology at the University of Turin Medical School. His current scientific interests are the placebo effect across diseases, pain in dementia, and intraoperative neurophysiology for mapping the human brain.
Fabrizio Benedetti, MD
Department of Neuroscience
Clinical and Applied Physiology Program
University of Turin Medical School
Corso Raffaello 30
10125 Turin
Italy
fabrizio.benedetti@unito.it
Homepage
|
