| No. 66 Hormones |
|
Where in the brain do estrogens act and what do they do? Estrogens are generated from cholesterol via testosterone in the ovaries, but also by the brain and by body fat deposits in both males and females [1]. Gonadal testosterone as well as adrenal androgens are the sources of estrogen formation in fat tissue and brain (fig. 1). Both males and females express intracellular ERs throughout the body and in many parts of the brain (see below). 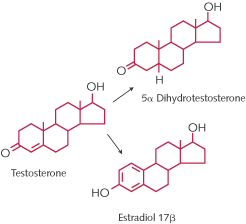 Fig. 1.
Testosterone is converted to estradiol via aromatizing enzymes and to 5-alpha dihydrotestosterone via 5-alpha reductase. We have known for more than 40 years that estrogens target the brain of experimental animals. The first animal studies focused on estrogen actions on the hypothalamus affecting ovulation and reproductive behavior. Only recently has it become apparent that estrogens exert many actions on brain areas that are important for learning, memory, emotions, and affective state as well as motor coordination and pain sensitivity [2]. Table 1 summarizes some of these estrogen effects.
Where in the brain do estrogens exert these actions? As summarized in table 2, many widely projecting neural systems such as the basal forebrain cholinergic system, the midbrain serotonin and dopamine systems, and the brainstem cholinergic and noradrenergic systems are targets of estrogen action (fig. 2). In addition, the hippocampus, a structure important for declarative, episodic, and spatial learning and memory, is also responsive to estrogens (fig. 3), as are cerebral blood vessels and glial cells. 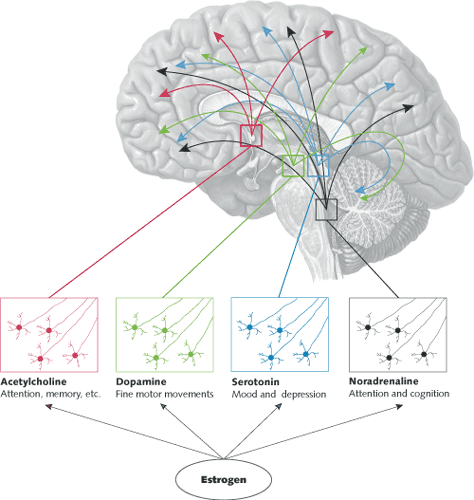 Fig. 2.
Several neurotransmitter systems which project widely into the forebrain are influenced by circulating estrogens. Basal forebrain cholinergic neurons regulate attention and other functions. Midbrain and brainstem monoamine-producing cells (serotonin, dopamine, and noradrenaline) are involved in attention, mood, memory, motor activity, and fine motor skills. 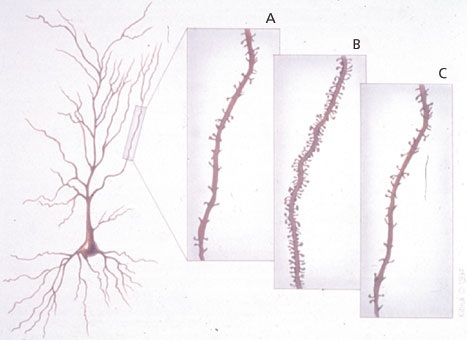 Fig. 3.
Spine synapses on hippocampal pyramidal neurons, which are sites of excitatory neurotransmission important for learning and memory, are replaced during the 4- to 5-day estrous cycle of a female rat under the influence of estradiol and progesterone. Hippocampal-dependent memory processes are affected in parallel with the increases and decreases in synapse density. A. Diestrus – the beginning of the cycle when estradiol levels are low. B. Proestrus – the day when ovulation occurs and sexual receptivity is shown. C. Estrus – the day after proestrus, when the system is beginning to reset itself for the next cycle. ERs and molecular mechanisms of estrogen action in the brain Intracellular ERs were first identified in the 1960s by binding of tritiated estradiol: they are proteins that bind to DNA in the cell nucleus when estrogens are bound to them (fig. 4). Found initially in the reproductive tract, putative ERs were subsequently identified in the pituitary gland and hypothalamus. These were studied for many years because they were the most obvious and also the most obviously related to estrogen actions on reproduction. More recently, estrogen-sensitive brain regions have been identified, not so much because of the localization of cell nuclear ERs but because of the effects that estrogens produce. This, in turn, has led to the recognition that estrogens produce their effects via a variety of intracellular mechanisms and sites of action. With the development of antibodies to ERs and ER cloning, intracellular ERs themselves or their mRNAs could be measured by immunocytochemistry and in situ hybridization histochemistry. The classical intracellular ER is ERα, while a new form, ERβ, has recently been identified and cloned: it is found in some tissues and brain regions not previously known as estrogen targets, while also existing with ERα in other tissues and brain regions. Alongside these discoveries with intracellular ERs, studies of estrogen effects on nerve cell activity and neuroprotection have uncovered rapid actions of these hormones that, either because they are so fast or from what we know of their structure-activity profile in relation to the specificity of known intracellular ERs, cannot involve activation or repression of gene expression (fig. 4). These ‘nongenomic’ actions of estrogens operate in many cases at or near the cell surface and affect the excitability of nerve and smooth muscle cells and the movement of the sodium, potassium, and calcium ions that create a nerve impulse. However, we know very little about the molecular characteristics and the mechanism of action of these ‘nongenomic’ receptors in cell membranes, though several recent studies have shown that intracellular ERs of the nuclear type may be expressed in small numbers at or near the cell surface where they could regulate second-messenger systems [3]. 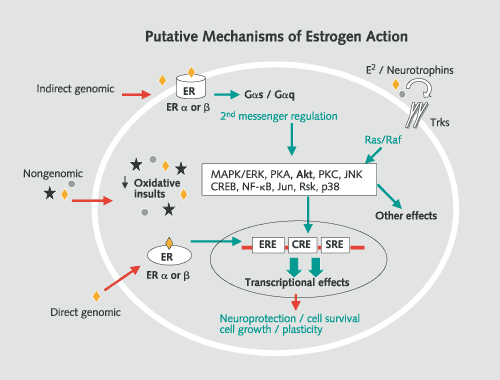 Fig. 4.
Putative mechanisms of estrogen action affecting cell growth, neuroprotection, and cell survival, and structural plasticity. In the direct genomic mechanism, the nuclear form of ERα or ERβ associates with either the estrogen response element (ERE) on DNA or fos/jun heterodimers that bind, in turn, to AP-1 sites on DNA. Indirect genomic mechanisms include the activation of an estrogen receptor linked to second-messenger systems, such as protein kinase C (PKC), cyclic AMP and protein kinase A (cAMP/PKA) and mitogen-activated protein kinase (MAPK/ERK), converging with the genomic pathway. In one of these pathways, Ras activates Raf, which leads to sequential phosphorylation and activation of MAPK/ERK. Activated ERK then translocates into the nucleus to interact directly with nuclear transcription factors (e.g., CREB, cfos/cjun), and indirectly through the activation of intermediary signaling proteins (e.g., Rsk, p38, JNK) to bind to the DNA regulatory regions, cAMP response element (CRE), and serum response element (SRE). Neurotrophins and estrogens may influence each other’s actions by regulating receptors and/or ligand availability through reciprocal regulation at the genomic level. Nongenomic estrogen effects at high concentrations involve antioxidant effects not mediated by known intracellular estrogen receptors. Terminology: ERE, AP-1, SRE, and CRE are regulatory regions in DNA sequences that are recognized by specific gene regulatory proteins. ERE is recognized by estrogen-ER complexes; AP-1 is recognized by fos/jun heterodimers; CRE is recognized by phospho-CREB (phosphorylated by PKA in response to a rise in cAMP levels); SRE is recognized by the SRF-Elk-1 complex phosphorylated by MAPK/ERK. MAPK/ERK migrates from the cytoplasm to the nucleus and phosphorylates Elk-1, thereby activating it to turn on transcription of the fos gene. MAPK/ERK and PKC can phosphorylate jun protein, which combines with the newly formed fos to form heterodimers that ultimately bind to AP-1. Estrogen effects in the hippocampus: genomic and nongenomic effects Estrogen treatment increases dendritic spine density on CA1 pyramidal neurons in the female rat hippocampus, whereas progesterone treatment acutely enhances spine formation, but causes the down-regulation of estradiol-induced synapses over the next 24 h (fig. 3) [4]. The mechanism of estrogen action involves both genomic and nongenomic effects. Moreover, estrogens do not act alone, and ongoing excitatory neurotransmission involving NMDA receptors is required for synapse induction: estradiol treatment increases NMDA receptor density in the CA1 region of the hippocampus by a mechanism that may not involve transcriptional regulation by estradiol. Where are the ERs that mediate these effects? Adult CA1 pyramidal cells of the dorsal hippocampus do not express detectable cell nuclear ER. Instead, immunocytochemistry for ERα showed cell nuclear ERs in sparsely distributed interneurons in the CA1 region and other regions of Ammon’s horn. Beside cell nuclear ERs, evidence is increasing for nonnuclear ERs that interact with second-messenger pathways. Using electron microscopic immunocytochemistry, ERα-immunoreactivity (IR) was detected in dendritic spines of principal cells, where it was often associated with spine apparati and/or postsynaptic densities, suggesting that estradiol might act locally to regulate calcium availability, phosphorylation, or protein synthesis. Other ERα-IR was found in unmyelinated axons and axon terminals containing small synaptic vesicles, where it may be involved in regulating neurotransmitter release. The close association between ERα-IR and dendritic spines supports a possible local, nongenomic role for this ER in regulating dendritic spine density via second-messenger systems (see fig. 4, 5). How can nuclear and nonnuclear actions of estradiol work together in the hippocampus (fig. 5)? Acting on the ERα-containing inhibitory interneurons, estradiol decreases GABA inhibition of excitatory neurons where the new spine synapses are generated. Concurrently, ERs in dendritic spines may be associated with the activation of mRNA translation from polyribosomes or endomembrane structures found in spines. In addition, other second-messenger signaling effects might include the phosphorylation of neurotransmitter receptors or ion channels. ERs in certain presynaptic terminals might modulate neurotransmitter release or reuptake. Moreover, ER-mediated activation of second-messenger systems in dendritic spines and presynaptic endings might lead to retrograde signal transduction back to the cell nucleus, providing another pathway through which estradiol could regulate gene expression. 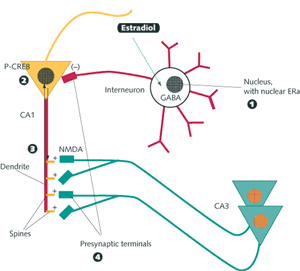 Fig. 5.
In the rodent hippocampus, estrogens operate via both genomic and nongenomic ERs to regulate the formation of excitatory spine synapses. (1) Estradiol works via genomic ERs in inhibitory interneurons to depress GABA activity. (2) Estradiol regulates the phosphorylation of CREB via a second-messenger pathway in CA1 pyramidal neurons where spine formation occurs, and pCREB is involved in regulating expression of genes that remain to be determined. (3) Estradiol regulates local protein synthesis in dendrites from mRNA that has been transported into the dendrites; the Akt signaling pathway is involved. (4) Estradiol may also regulate the release of neurotransmitters such as acetylcholine from presynaptic terminals via ERs located in the terminals. Aging female brain With an increasing life expectancy, women may live a substantial part of their lives with greatly reduced estrogen levels after the menopause. For many women, hot flushes are the most dramatic and noticeable consequence of ovarian hormone loss. But there are other less obvious and more gradual changes, such as loss of bone calcium and osteoporosis, that have led many women to take hormone replacement therapy (HRT) at the menopause. Furthermore, the loss of protection of coronary arteries by estrogens, increasing the risk for cardiovascular disease, has also reinforced the value of HRT. The brain also suffers from the loss of this circulating hormone. There are two aspects to this loss, namely, reversible changes in cognitive function and an irreversible decline associated with the onset of dementia. Loss of estrogens following suppression of ovarian function with gonadotropin-releasing hormone agonists, or the loss of ovarian hormones as a result of surgical and natural menopause, leads to generally reversible decreases in declarative memory and motor coordination that respond to estrogen replacement therapy. Estrogen actions in the hippocampus are suspected to underlie the declarative-memory deficits. A long-term consequence of estrogen loss at menopause is an increased risk for Alzheimer’s disease that can be reduced by estrogen replacement therapy, although treatment with estrogens once the disease is clearly established apparently has no beneficial effect [5]. Estrogens could protect the brain from neurodegeneration in at least two ways. As discussed above, estrogens maintain the function of key neural structures such as the hippocampus and basal forebrain and the widely projecting dopaminergic, serotonergic, and noradrenergic systems (tables 1, 2). As estrogen levels decline over the menopause, these systems and the cognitive and other behavioral processes that depend upon them also decline, at least functionally, but appear to respond to estrogen replacement. Estrogens not only maintain function but may also confer resilience against neural damage maintaining synaptic connections and promoting the activity of these important neural systems. Estrogens may also directly block the actions of neurotoxic agents or inhibit their generation. The A ring of the estrogen molecule appears to have special properties with respect to the formation of free radicals, and special protective effects on cells in culture deprived of serum or exposed to free radical generators. In addition, estrogen treatment of cultured nerves decreases formation of the toxic form of the beta-amyloid protein. Estrogen treatment also interferes with the toxic effects of the beta-amyloid protein and the HIV coat protein, gp120, both of which act via free radical generation. Hormone replacement therapy The recent termination of the arm of the HRT trial of the Women’s Health Initiative (WHI) that involved administration of the estrogen-progestin combination known as PremPro (Premarin and Provera) brings the issue of HRT into sharp focus [6]. The purported benefits of HRT for the brain include improved cognitive function, motor coordination, and reaction times, protection from brain damage due to stroke, and reduced risk of Alzheimer’s disease. It is important to note that the estrogen-only arm of the WHI trial was not terminated, thus focusing attention not on the efficacy of estrogen replacement per se, but rather on the failure of PremPro to have beneficial effects for cardiovascular disease. Indeed, the progestin component of the HRT, in this case medroxyprogesterone acetate (Provera), and the way it was administered appear to have been the major culprits. Because Provera is a synthetic progestin, it interacts with glucocorticoid and androgen receptors as well as progesterone receptors. Moreover, in the most common form of HRT, the combination of Premarin and Provera were given concurrently, contrary to the natural secretion pattern of estradiol and progesterone which are sequential. Thus the failure of the PremPro form of HRT is an indictment of Premarin and Provera and their concurrent administration, rather than a failure of HRT in general, a distinction that, unfortunately, has not emerged in much of the press coverage of the termination of the WHI trial. The distinction between the actions of Provera and progesterone are quite apparent in studies on nerve cells and brain tissue. In nerve cells in culture, estradiol, together with progesterone and 19-norprogesterone, exerts protective effects against toxicity by glutamate; in contrast, Provera did not protect against glutamate neurotoxicity and attenuated the neuroprotective effects of estradiol. For the serotonin system, estrogen administration increases synthesis of the neurotransmitter serotonin by inducing the rate-limiting enzyme, tryptophan hydroxylase, in midbrain raphe nucleus neurons; progesterone administration after estrogen priming does not alter this induction, but Provera treatment reverses the estrogen effect [7]. An alternative to replacement with estradiol and progestins is the use of so-called selective estrogen response modulators (SERMs), which have the advantage of antagonizing proliferative effects of estrogen on estrogen-dependent cancer and are, at the same time, partial agonists for some estradiol effects. For example, the SERM raloxifene mimics estradiol in inducing tryptophan hydroxylase in the midbrain raphe, and another SERM, CI-628, mimics estradiol effects to induce a rate-limiting enzyme for acetylcholine formation, choline acetyltransferase, in the basal forebrain. At the same time, SERMs antagonize some effects of estradiol: e.g., CI-628 blocks estradiol induction of new spine synapses in the hippocampus of the female rat. Therefore, SERMs may enhance some, but not all, of the beneficial actions of estradiol on the brain and other systems. Comparison with the male brain Males show a lesser decline of gonadal function with increasing age and do not suffer from a substantial loss of androgens, and so we know much less about the effects of androgen or estrogen replacement therapy on cognitive function or protection from Alzheimer’s disease in men. In one study, androgen reduced beta-amyloid formation. However, since in animal models there are developmentally programmed sex differences in many of the neural systems that respond to estrogens (tables 1, 2) and androgens, estrogen and androgen actions in men and women are unlikely to be equivalent. Although the normal physiological effects of estrogens on the widespread estrogen-responsive systems of the brain (tables 1, 2) may show sex differences, the antioxidant and related neuroprotective effects of estrogens summarized above will probably not differ between men and women. Considering the fact that androgens may themselves have beneficial effects on the brain, and that testosterone is converted not only to 5 alpha-reduced androgens but also to estrogens in the brain and fat tissue, testosterone replacement in males may bring benefits, even if the estrogens produced by aromatization may not do exactly the same things in males as estrogens in females. Future challenges Estrogen effects on the brain must now be regarded in a broad arena that encompasses many aspects of brain function, including mood, motor control, pain, higher cognitive processes, and the new domain of neuroprotection. Recognition of multiple ER types and intracellular mechanisms of action has opened the door to the study of estrogen effects in brain regions not previously recognized to be estrogen sensitive. The multiple mechanisms of estrogen action present new opportunities and challenges for pharmaceutical strategies directed toward developing agents targeted to specific processes such as reduction of free-radical-related damage in the brain while minimizing the risks associated with estrogen replacement therapy. At the same time, study of androgen actions and the potentially beneficial effects of androgens may help to counter the loss of cognitive function and risk for dementia in aging males. Study of gender differences in the actions of gonadal hormones has now moved beyond strictly reproductive functions into their roles in major functions of the nervous system in health and disease.  Bruce S. McEwen is the Alfred E. Mirsky Professor and Head of the Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology at Rockefeller University, New York, NY. His laboratory studies the impact of stress and stress hormones on the brain and immune function, the nonreproductive actions of sex hormones, and brain sexual differentiation. As past president of the Society for Neuroscience and the International Society of Neuroendocrinology, Dr. McEwen is on the editorial board of numerous journals including the Karger publications Neuroimmunomodulation, Developmental Neuroscience, and Interdisciplinary Topics in Gerontology.
Bruce S. McEwen, Ph.D. Head, Harold and Margaret Milliken Hatch Laboratory of Endocrinology The Rockefeller University, Box 165 1230 York Avenue New York, NY 10021 USA Phone: (1) 212 327 8624 Email: mcewen@mail.rockefeller.edu Homepage: http://www.rockefeller.edu/labheads/mcewen/mcewen-lab.html |
|||||||||||||||||||||
| Close Window |